GST : 29AACCD4802G1ZV
About Us
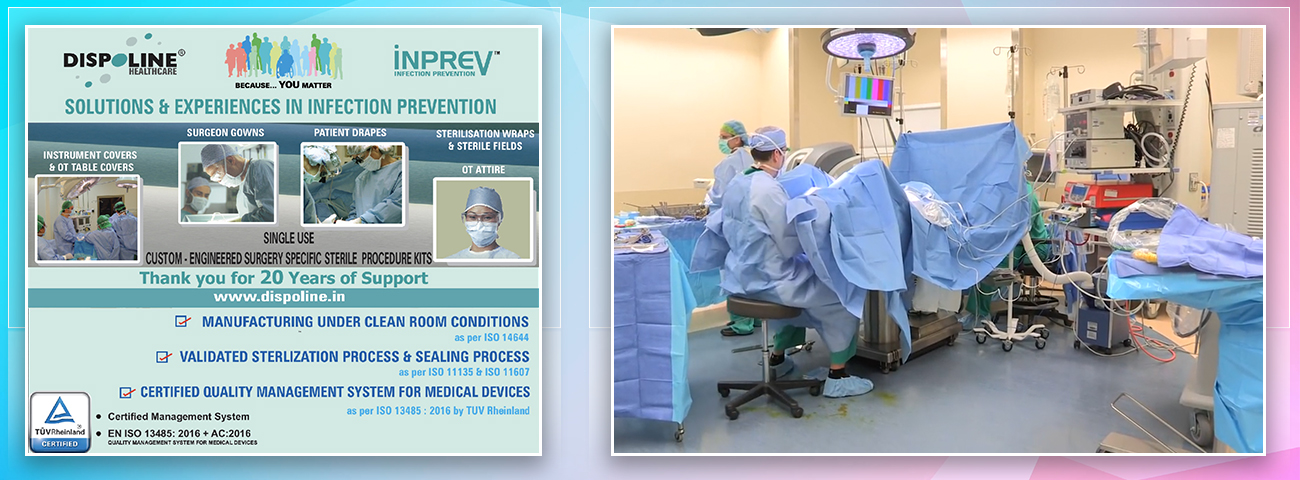
Dispoline Inprev- Solutions & Experiences in Infection Prevention.
Dispoline Inprev is a 20-year-old Infection Prevention company. An integrated manufacturing company making Single-Use Products for the Healthcare industry.
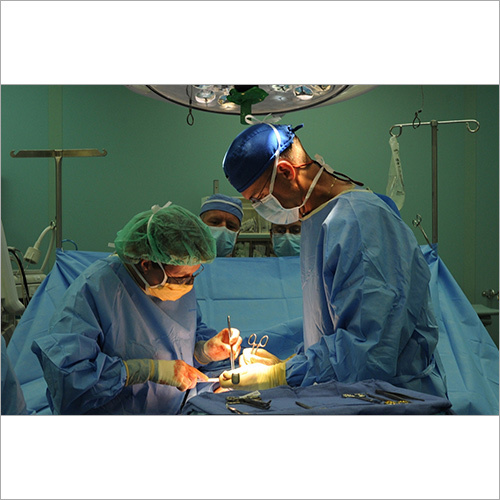
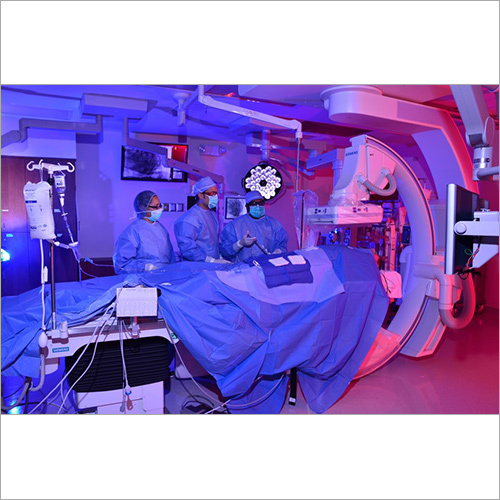
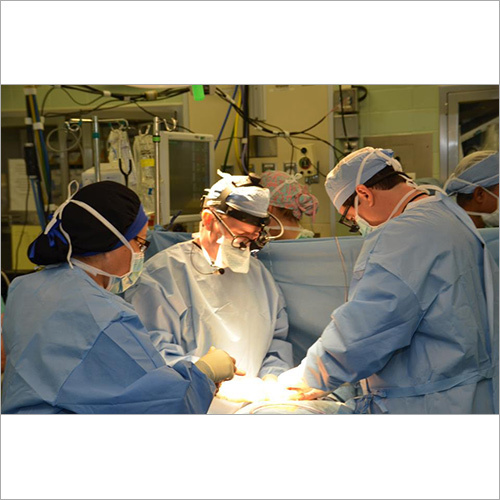
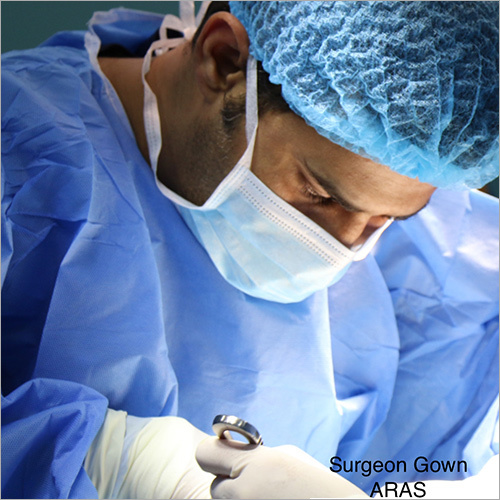
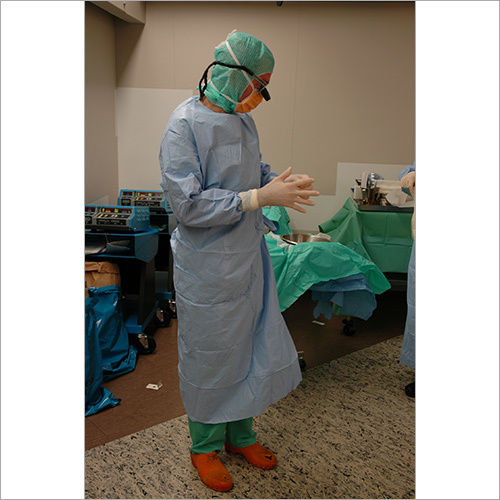
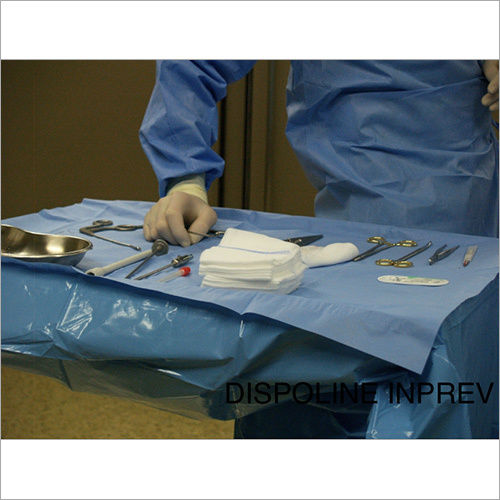
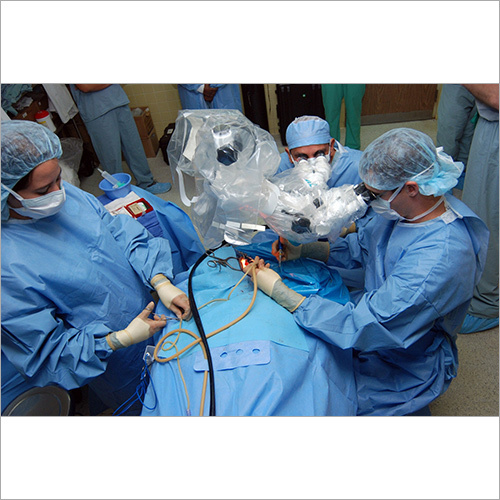
We focus on Surgery Specific Procedure Kits- consisting of Surgeon Gowns, Surgical Patient Drapes, and Instrument covers. (Custom engineered to your requirements)
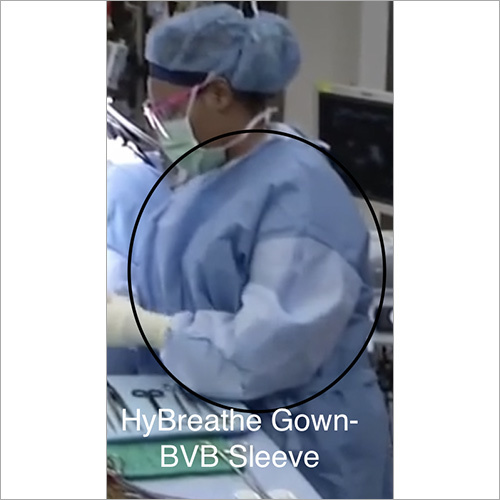
Other products include- Breathable Viral Barrier Gowns, ARAS Surgical Gowns, Surgical Drapes, Surgical & Medical Attire, Small Procedure Kits, as well as Sterile Barrier Systems.
Quality Management :
We have been certified by- M/s TUV Rheinland Gmbh - along with Accreditation from DaKKS Gmbh for EN ISO 13485:2016- Quality Management System for Medical Devices. Our sterilization process is also validated as per EN ISO 11135.
We are registered under Central Drugs Standard Control Organisation (CDSCO) for Medical Devices. Our Current Registration No. is Dispol-Benga-KA/M/MD/003350
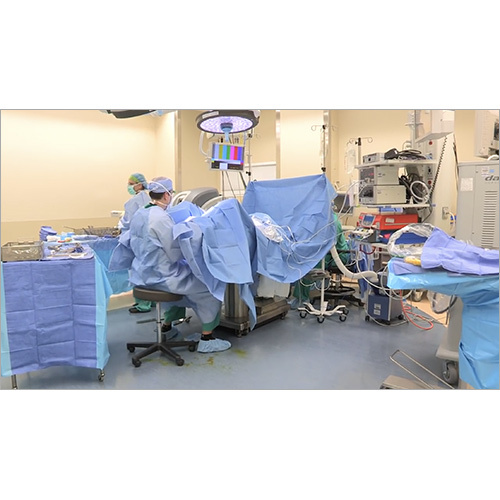
We follow a host of other Global Standards and Best Practices. These products are made at modern, compliant, manufacturing plants with procedure-specific cleanrooms.
Our Values
- Integrity & Ethics- Our commitment to ethics and integrity underpins all of our core values.
- Relationships- All of the people with whom we work, we strive to develop mutually beneficial partnerships- Customers, Principals, Vendors, Employees, and the general public with whom we hope to build long-term relationships based on the Win-Win philosophy.
- Innovation- We try to increase the value, quality, and effectiveness of our solutions for our clients by employing new approaches. We plan to do so by incorporating our customers, principals, workers, and all vendors in order to guarantee that innovative products/solutions that provide value to our customers are provided.
- Education- Because change is constant, we work hard to keep our customers, employees, and other stakeholders informed of any information of relevance. We seek to achieve this through ongoing education programmes led by subject specialists.
Our Mission
Our mission is to provide to the healthcare industry Single use Infection Prevention Products which are High Quality, Cost Effective, Increase safety and Aid productivity. We will continue to develop products that provide superior value- through innovation and execution that is customer centric. We intend to integrate our broad resources to deliver innovative solutions, which create greater value for our customers.
Why Us?
Manufacturing in advanced production facilities with Process Specific Cleanrooms and Controlled Environmental Conditions.
- The Sterilization process has been verified in accordance with EN ISO 11135 standards, and a sterilization protocol has been designed to deliver that the process consistently gives a Sterility Assurance Level 10-6 (SAL) to the product. Each batch of sterilization is sent with a set of 16 Biological Indicators inside a PCD for routine monitoring of the sterilization process. (1.5 BIs per Cubic metre) The BIs are then incubated for 48 hours at 37 degrees Celsius before the product is being released. Internal and external chemical indicators are included in all packets to allow for a visual verification of ETO exposure. Internal chemical indicators are peel-off labels that have batch and production information printed on them so that they may be transferred to patient records for traceability.
- Pouch packaging complies with EN 868 and is obtained from a company that meets EN ISO 13485 and ISO 11607-1 standards.
- Packaging Sealing process is carried out using high-end machinery with online printing to ensure traceability protocols are met. The sealing procedure is validated as per EN ISO 11607-2 and has an integrated system to ensure essential parameters such as temperature, pressure and time.
- Medical fabrics are acquired from the top manufacturers across the world and meet a variety of standards depending on product uses and client specifications. AAMI PB70, EN 13795, EN ISO 11607, and other standards are applied.
- Batch/Run Wise Monitoring of Critical Quality Parameters is performed on our Medical Fabrics, and the results are recorded.
- Cytotoxicity and Skin (Dermal Irritation) Tests are also performed on the medical fabrics.
- Third party Testing by Accredited labs-
- Residual tests for Ethylene Oxide, Ethylene Chlorohydrin & Ethylene Glycol as per ISO 10993-7
- Sterility Tests done as per ISO 11737-2.
- The bioburden analysis is carried out in accordance with ISO 11737-1.
- Usage based sealing methods are followed-
- Ultrasonic Sealing, Impulse heat sealing, Hot Melt Glue, Adhesive Tapes etc.
- In terms of the product and raw materials, all traceability protocols are followed.
 |
DISPOLINE INDIA PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |

 Send Inquiry
Send Inquiry